Note: 35-009 (S) & 35-009NS (NS) are the equivalent Protex®4 replacement for SMS drape 28-369 (S) & ST 0410 (NS).
Product Details
Size
254(230)cm x 317cm
Units Per Inner
-
Inners Per Carton
-
Carton quantity
12 pcs
Sterile
Sterile
Use
Colour
Blue
More Information
Gynaecological Drapes
Multigate Gynaecological Drapes cover the full range of relevant procedures and are known for their exceptional fluid management and infection control. They are cost effective and reduce the risk of contact with body fluids for clinicians and patients.
Relevant Procedures
- 1244 Other excision procedures on ovary
- 1251 Salpingectomy
- 1258 Other procedures on fallopian tube
- 1259 Examination procedures on uterus
- 1260 Insertion or removal of intrauterine device
- 1263 Destruction procedures on uterus
- 1265 Curettage and evacuation of uterus
- 1266 Excision of lesion of uterus
- 1268 Abdominal hysterectomy
- 1269 Vaginal hysterectomy
- 1275 Destruction procedures on cervix
- 1283 Repair of prolapse of uterus, pelvic floor or enterocele
- 1297 Procedures for reproductive medicine
- 1299 Other procedures on female genital organs
Instructional Videos
Protex®4 - Advanced Drape Protection System
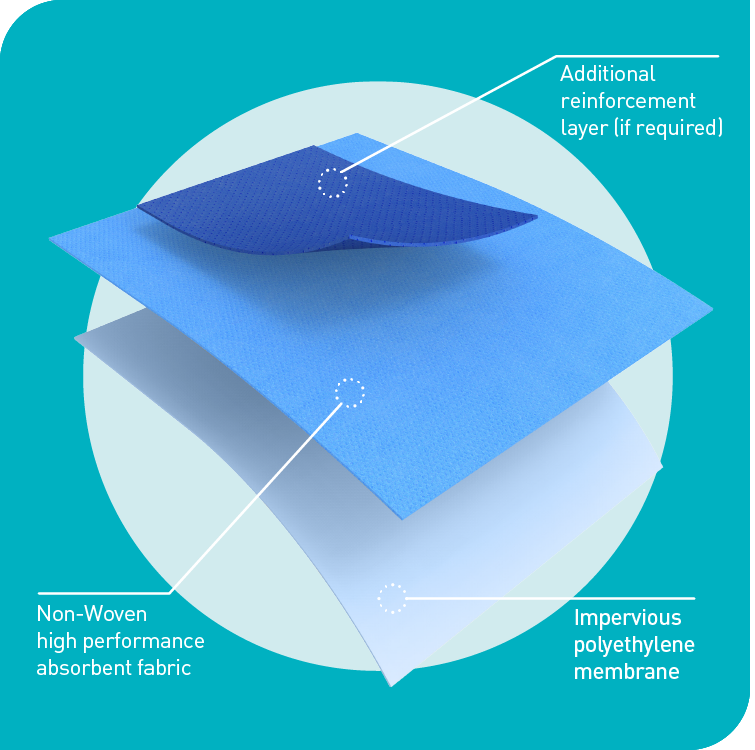
Advanced materials technology that improves drape imperviousness and absorbency to deliver unbeatable patient and surgical team protection.
Protex®4 drapes are made from a pliable yet tough impervious bi-laminate material supported by an additional ultra-reinforcement layer to further boost absorbency if required. They are designed to eliminate bacterial transfer and deliver maximum absorbency and strikethrough prevention.
Intellectual Property rights such as patents, patent applications, registered designs etc. apply to this product group.
For details refer to applications: 201613307, 201712340, 201713600, 201713601, 201713962.

.png?height=600)